
CeraPlus™ Skin Barrier Caused Significantly Less Disruption to the Skin Compared to SenSura Mio Skin Barrier**1
CeraPlus™ skin barrier with Remois Technology* demonstrated to minimize transepidermal water loss (TEWL) and skin irritation compared to SenSura Mio Skin barrier.**1
When you remove an ostomy barrier, what else are you peeling away?
When an ostomy barrier is removed, cells from the outermost layer of skin are also removed along with it. This damage to the skin allows increased moisture from inside the body to pass through into the environment. Escaping moisture is called transepidermal water loss (TEWL) and can be assessed in a lab with a tool that measures evaporation. A lower TEWL value indicates better skin integrity.1-6

The CeraPlus™ skin barrier observed to be more effective than SenSura Mio skin barrier at protecting skin from the damage of adhesive removal1
Minimizing TEWL
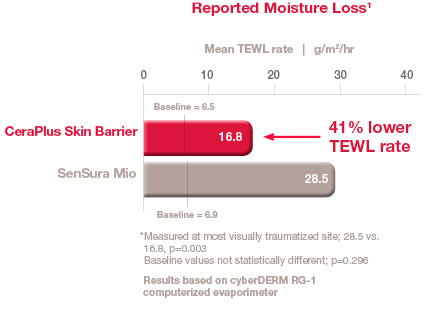
TEWL was 41% lower with the CeraPlus skin barrier than SenSura Mio skin barrier.1
Minimizing skin irritation
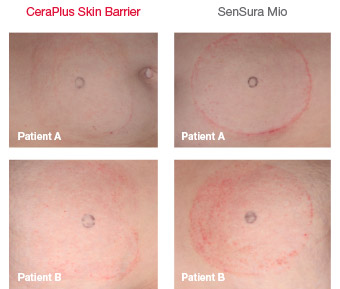
Images taken on final day of participation
CeraPlus skin barrier is significantly less disruptive to the skin compared to SenSura Mio skin barrier.1
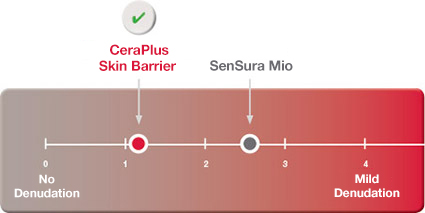
Protecting Skin From Damage
CeraPlus™ skin barrier provided better protection against the damaging effects of repeated adhesive removal than SenSura Mio.1
CeraPlus™ Skin Barrier
Helps maintain healthy skin from day one.
Ceramide-infused formulation helps protect against damage, dryness, and itching.
The CeraPlus™ skin barrier comes in a range of fit options including one-piece, two-piece, flat, firm convex, soft convex, tape border, and tapeless.
The ADVOCATE study showed how the CeraPlus™ skin barrier reduces cost of care and reduces PSCs
Fit + Formulation is the inspiration behind CeraPlus™ skin barrier
*Remois is a technology of Alcare, Co., Ltd.
**SenSura Mio is a trademark of Coloplast AJS and its affiliates.
References:
- Grove G, Houser T. Sibbald G, Salvadalena G. Measuring epidermal effects of ostomy skin barriers. Skin Res Technol. 2019; 25:179-186. https://www.ncbi.nlm.nih.gov/pubmed/30387538.
- Grove GL. Techniques for assessing the vulnerability and repair capacity of human skin in vivo. In: Kligman AM, Klemme JC, Susten AS, eds. Am J Ind Med. 1985; 8:483-489.
- Cutting RF. Impact of adhesive surgical tape and wound dressings on the skin, with reference to skin stripping. J Wound Care. 2008:17:157-162.
- Grove GL, Zerweck C, Houser T, Smith GE, Koski NL A randomized and controlled comparison of gentleness of two medical adhesive tapes in healthy human subjects. J Wound Ostomy Continence Nurs. 2013; 40:51-59.
- Murayama RI, Taylor MG, Oamia J, Grove GL. Preliminary studies on the relationship among peel force, quantitative measures of skin damage and subjective discomfort. Skin Res Technol. 2008:14:478-483.
- Nybaek H, Lophagen S, Karlsmark T, Bang Knudsen D. Jemec GBE. Stratum corneum integrity as a predictor for peristomal skin problems in ostomates. Br J Dermatol. 2010; 162:357-361.
- Nichols T. Houser T, Grove G. Comparing the skin stripping effects of three ostomy skin barriers infused with ceramide, honey or aloe. J Stomal Ther Au. 2019: 39:14-18.

 Call Us
Call Us  Sign Up
Sign Up  Email Us
Email Us 